Building Trust from the Source: A Comprehensive Raw Material Control System
How we implement end-to-end quality assurance before processing even begins
Source Control & Raw Material Inspection: Intercepting Risks Before the Production Line
Deep Dive: How We Build Trust from the Source
Ever wondered what truly lies behind a certificate or a quality claim? In this edition of Angfa Connect, we pull back the curtain on the foundational system that makes everything else possible: our comprehensive raw material control regime. Explore the science, protocols, and data-driven decisions that occur long before processing begins, and see how this depth of oversight translates into uncompromising safety and consistency for your supply chain.
When you click to read this page, it means you share our belief: exceptional seafood quality cannot be "added" during processing; it must originate from an impeccable starting point (fresh raw materials). This article aims to elaborate on the core commitment of our Quality Control page: Source Control & Raw Material Inspection.
1. Species Traceability & Sustainable Sourcing Verification: Ensuring Authenticity and Responsibility for Every Batch
The deep management system behind species authenticity, freshness, and microbiological safety. Many species look extremely similar in their juvenile stages, for example: Loligo duvauceli, Uroteuthis edulis, Loliolus beka. At Angfa, our quality firewall activates even before raw materials arrive at the dock, through a scientific verification process that establishes a solid foundation for the stability of your supply chain and the safety of end products.
To prevent prevalent species mislabeling and fulfill sustainable sourcing commitments, for high-risk products like baby squid that are prone to species confusion, we commission independent laboratory Eurofins to conduct species identification using DNA barcoding technology. This not only ensures that the products you receive are 100% consistent with contract specifications, eliminating commercial fraud risks, but also provides irrefutable scientific evidence for our own supply chain transparency.

DNA test report sample showing species identification with Latin names and match percentages
2. Quantification of Core Freshness Indicators and Proactive Control
"Freshness" is a concept that requires data definition. By monitoring key chemical indicators, we transform sensory judgment into measurable, controllable scientific parameters.
- TVB-N: The Scientific Scale Defining Freshness: Total Volatile Basic Nitrogen (TVB-N) is an internationally recognized core indicator of fish freshness. We strictly adhere to an internal acceptance standard of ≤15mg/100g (this standard is stricter than many common market requirements), ensuring raw materials are in optimal freshness condition. All batch TVB-N test data is entered into our system for trend analysis and supplier performance evaluation.
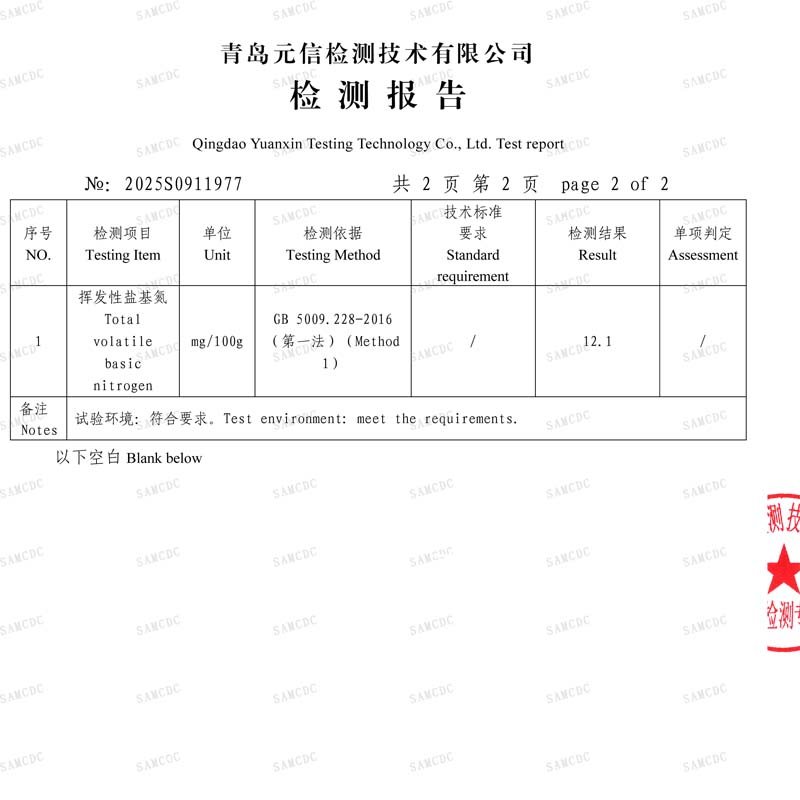
TVB-N Result
- Multi-Indicator Collaborative Monitoring: In addition to TVB-N, and based on requirements from clients in different regions, we also include pH value, Trimethylamine (TMA), and others in our screening scope, forming a comprehensive freshness evaluation matrix that ensures the best flavor, texture, and shelf life for finished products right from the source.
3. Proactive Microbiological Risk Prevention: Establishing a Safety Baseline from the Source
We understand that safe processing begins with safe raw materials. To proactively prevent risks, rather than merely meeting final product standards, we establish a microbiological safety baseline at the raw material reception stage that far exceeds conventional requirements.
Pathogen Screening Based on Regulatory Safety Objectives: Guided by the principles of final food safety requirements in regulations such as EU (EC) No 2073/2005, we screen raw materials for key foodborne pathogens like Salmonella, Listeria monocytogenes (where applicable), ensuring high-risk pathogens do not enter the production process.
Hygiene Indicator Monitoring Beyond Regulations: To provide earlier warning of hygiene risks, we additionally monitor levels of key hygiene indicator organisms like Enterobacteriaceae and Total Aerobic Count. This provides scientific data for assessing the initial hygiene status of raw materials and suppliers' hygiene control levels, forming an important part of achieving proactive quality management.
Any positive result leads to rejection of the entire batch of raw materials and triggers the supplier corrective action process.
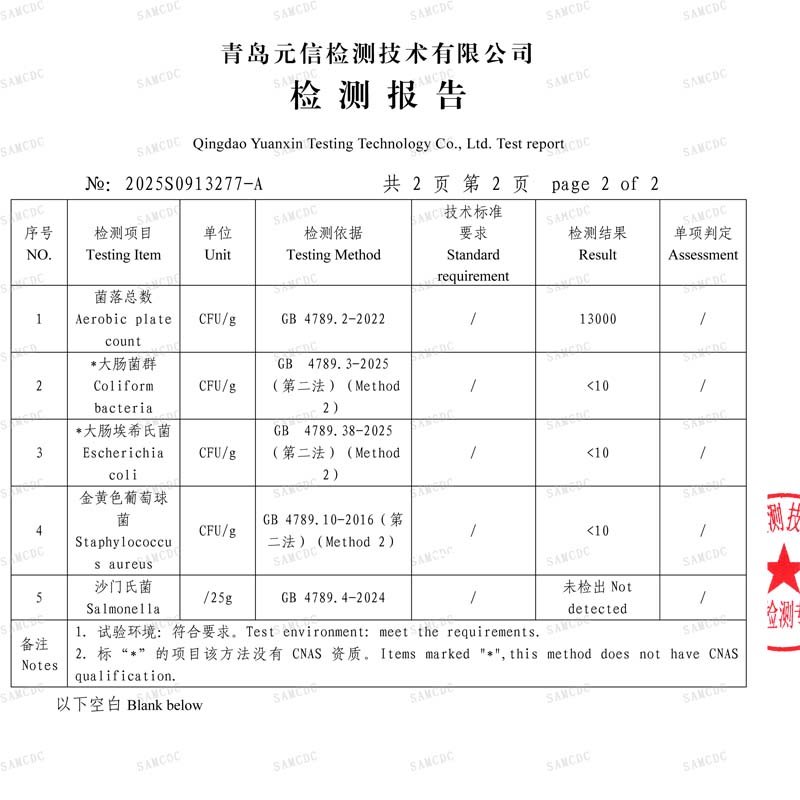
Microbiological test report showing screening results*
*Raw materials should comply with the extended requirements of finished product regulations. When inspecting raw materials, companies typically refer to the standards established for finished products in Regulation (EC) No 2073/2005 particularly its process hygiene criteria as the basis for assessing the hygiene status of raw materials. This regulation serves as the core EU legislation on microbiological criteria for foodstuffs. Additionally, compliance is required with the EU’s general food hygiene regulation, (EC) No 852/2004, and the specific hygiene rules for food of animal origin under Regulation (EC) No 853/2004.
Extended Environmental Monitoring: We believe monitoring should extend beyond the raw materials themselves to include the hygiene status of raw material transport vehicles, ensuring complete cold chain and hygiene safety from point of origin to factory. These are all tasks we strive to accomplish!
4. Beyond Inspection: Systematic Supplier & Risk Management
Inspection is a verification tool, not a management objective. Through systematic management, we seamlessly integrate quality requirements into the entire supply chain.
- Documented Standards & Dynamic Assessment: All inspection activities are based on our detailed "Quality List of Squid Inspection & Quality Product Report", which integrates customer requirements, Chinese national standards, and regulations of target markets like the EU. Simultaneously, we drive continuous improvement by evaluating partners across multiple dimensions including quality, delivery, and compliance.
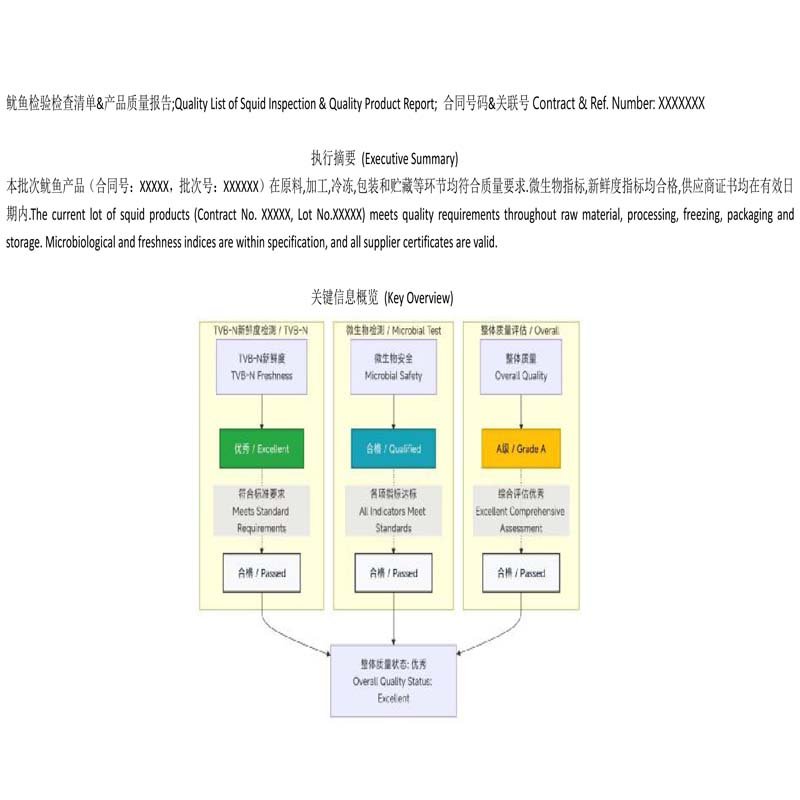
Sample of the Quality Product Report showing integrated standards and requirements
- Transparent Sharing & Collaborative Enhancement: Utilizing our self-deployed cloud services, we proactively share quality data and trend analysis with key suppliers, extending our quality management system forward and transforming "monitoring" into "empowerment", jointly building a more robust and resilient supply network.
Your Value & Our Commitment
What It Means for You:
- Reduced Commercial & Compliance Risks: Eliminates species fraud, avoiding subsequent trade disputes and brand reputation damage.
- Stable Product Quality: Consistent raw material freshness is fundamental to the stable taste and quality of your products.
- Simplified Supply Chain Management: We provide fully traceable verification documents (from DNA reports to microbiological tests) that can be directly used for your compliance audits, saving costs and management effort for secondary verification.
At Angfa, source control is not an isolated quality inspection procedure but the practice of our core philosophy: "Quality Begins at the Source". It enables the excellent performance of every subsequent process, ultimately ensuring that every product delivered to you carries reliability and trust.
Source Control & Raw Material Inspection | Source-to-Supply Excellence
Pathogen Screening Based on Regulatory Safety Objectives: Our raw material screening strictly adheres to the core requirements of EU food safety legislation, particularly Commission Regulation (EC) No 2073/2005 (on microbiological criteria for foodstuffs) and Regulation (EC) No 852/2004 (on the hygiene of foodstuffs). These regulations establish the primary responsibility of food business operators for food safety and mandate that control measures and microbiological criteria must be based on HACCP principles and scientific risk assessment to ensure that food does not contain microorganisms presenting an unacceptable risk to health.



